Professional products, now available for use at home
You can buy all our products from our Amazon store, ready for next day delivery.
CLINELL BIODEGRADABLE SURFACE WIPES – PACK OF 60 WIPES

Plastic-free surface wipes for everyday cleaning & disinfection, kill 99.99% of bacteria and viruses. Environmentally friendly, without compromising efficacy.
CLINELL BIODEGRADABLE HAND & SURFACE WIPES

One-step cleaning and disinfecting wipes for hands and surfaces in an easily transportable cup size tub, ideal for use whether at home or on-the-go.
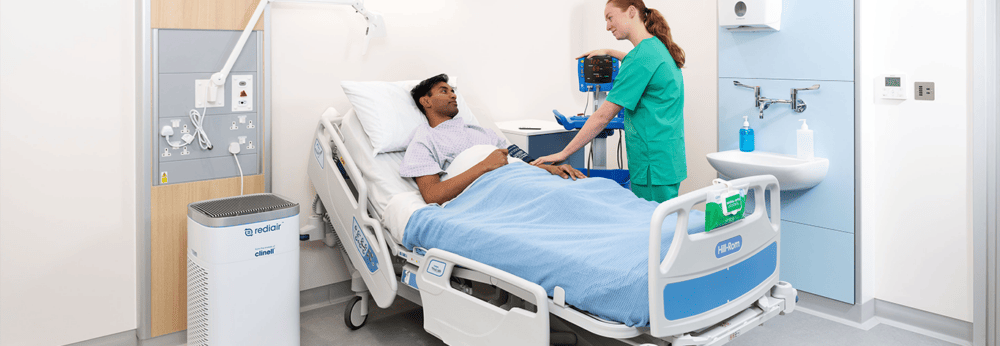
REDIAIR – PORTABLE INSTANT AIR FILTRATION UNIT

Instant air purification unit for poorly ventilated spaces, with dual HEPA 14 filters. Rediair is ultra quiet and captures 99.995% of airborne pathogens.
CLINELL HAND WIPES – BOX OF 20 PACKS X 8 WIPES

Clinell Hand Wipes clean and disinfects hands in one wipe, offering protection in just 30 seconds, making them perfect for when you’re on the move.
CLINELL UNIVERSAL MULTI-PURPOSE SURFACE WIPES – PACK OF 70

Clinell wipes to clean and disinfect surfaces in one easy step. 70XL wipes proven to kill at least 99.99% of germs, effective against the COVID-19 virus in 30 sec.
CLINELL ANTIMICROBIAL HAND WIPES 100 SACHETS

Convenient box of 100 individually-wrapped hand disinfectant wipes. Proven effective against all known enveloped viruses in 30 seconds.
CLINELL UNIVERSAL SURFACE WIPES – PACK OF 200 WIPES

Clinell Universal Wipes designed to clean and disinfect in a single step, proven to kill at least 99.99% germs and effective against the COVID-19 virus in 30 seconds.
CONTIPLAN ALL IN ONE CLEANSING CLOTHS – PACK OF 25 CLOTHS

All-in-one continence care value pack of incontinence cleansing cloths with barrier cream. Clean, moisturise and protect in one step. Clinically proven to reduce IAD.
Designed to protect you and your home from viruses and bacteria
CLINELL UNIVERSAL WIPES KILL AT LEAST 99.99% OF GERMS

DERMATOLOGICALLY TESTED

SAFE TO USE AROUND BABIES AND ANIMALS

SINGLE STEP CLEANING AND DISINFECTION
We're here to keep you safe
All our products are rigorously tested to provide highest quality and efficacy. Our patented near-neutral pH formula ensures exceptional level of protection and is proven to kill at least 99.99% of pathogens including Norovirus & MRSA.
Our Clinell Universal wipes are also proven effective against the COVID-19 virus on surfaces in a 30 seconds contact time.
Rediair - Instant air purification
The 5 principles of cleaning

Are Clinell Universal Wipes tested against the actual COVID-19 strain?
Can I use Clinell Universal Wipes on my hands?
Where can I buy Clinell Wipes?
Are your wipes safe to use on surfaces?
What are the benefits of Clinell Universal Wipes?
COVID-19: How it spreads and how we stop it
See which frequently touched surfaces require special attention, to stop the spread of viruses, including COVID-19.
Disinfecting safely: The five principles of cleaning
See the guidelines for effective cleaning, to ensure you disinfect the surfaces correctly and prevent further spread of germs.
About GAMA Healthcare
GAMA Healthcare was founded in 2004 by two doctors, Dr Guy Braverman and Dr Allen Hanouka, to help save and improve lives. Thanks to their passion and expertise, GAMA is now at the forefront of infection prevention, protecting people across the globe. Our Clinell Universal Wipes are the NHS’ number 1 most used disinfectant wipe, trusted by 9 out of 10 NHS hospitals.
Latest
Introducing HEXI HUB: A seamless transition in our product line
We’re pleased to announce an update to our product offering…
Innovative solutions for tackling Carbapenemase-producing Enterobacteriaceae (CPE) at King’s College Hospitals
King’s College Hospital NHS Foundation Trust, one of London’s largest…
Gloves Off: reducing unnecessary plastic waste during environmental cleaning and disinfection
In this blog, Dr Phil Norville discusses the momentum-gaining ‘Gloves…
Gloves Off: Navigating SDS sheets and skin safety claims in environmental decontamination products
In this blog, James Clarke (Head of R&D, Science &…
Help reduce staff sickness and improve air ventilation with Rediair
As the winter season approaches, concerns over staff sickness and…
Moments that Matter: celebrating the fundamentals of infection prevention
To celebrate the fundamentals of infection prevention, we’re exploring the…
Tackling surface contamination is key to controlling Candida auris
Candida auris is a key emerging threat to healthcare facilities…
Save money on air purification with Rediair: A cost-effective solution to expensive alternatives
A comparative evaluation of three different stand-alone HEPA-based air systems…
New study links 2-in-1 wipes with reduced in-hospital mortality
In today’s blog, we’ll be discussing a new publication from…
How Rediair can address NHS England’s new air filtration guidelines
In this week’s article, we discuss the newly developed UK…